Central Facilities and Services
Our CPA Central Facilities for Bioimaging, Bioanalytics and Mass Spectrometry are at the heart of our activities, providing sophisticated technology and expertise.
The facilities are extensively used by all CPA groups, collaboration partners as well as external TUM partners.
Contact for queries: cpa(at)tum.de
Analysis and Characterization of Biomolecules, e.g. quality control and identification of proteins, nucleic acids and small molecules. Characterization of their interactions, thermal stability and (enzymatic) activities.
Micro-Calorimetry: Isothermal Titration Calorimetry (ITC) & Differential Scanning Calorimetry (DSC)
Microcalorimeters enable the qualitative and quantitative characterization of biomolecule dynamics, stability or interactions. This include especially the analysis of proteins in terms of interactions with other molecules as well as changes in conformation such as protein unfolding. The technique requires only minimal assay development, no labelling and only low amounts of sample.
Typically used to:
- Determine protein stability (DSC)
- Characterize protein-protein or protein-compound interactions (ITC)
Analytical Ultracentrifugation (AUC)
Similar to an ultra-high-speed preparative centrifuge, an analytical ultracentrifuge sediment particles at very high g-forces but monitors them in real time by an integrated optical detection system. These optical systems (UV-Vis absorbance and Rayleigh interference or Fluorescence) enable precise observation of the matrix-free, solution behavior of the molecules. State-of-the-art software allows comprehensive (qualitative and quantitative) data analysis making AUC the most versatile and accurate technology available for determining the molecular weight, hydrodynamic and thermodynamic properties of proteins or other macromolecules.
Typically used to:
- Determine sedimentation coefficients and/or molecular weight of protein complexes
- Analyze mixtures of different protein complexes
Characterize protein-protein interactions
Preparative Ultracentrifugation (PUC)
Ultra-high-speed preparative centrifuge sediments particles at high g-forces. Using varying g-forces or specific density gradients this allows the separation of particles of different density or molecular weight.
Typically used to:
- Sediment aggregates and fibers
- Preparation of organelles
- Cell fractionation
Mass Photometry
Using the principles of interference reflection and interferometric scattering, mass photometry allows accurate mass measurement of single molecules in solution, label free, in their native state requiring very low amounts of sample.
Typically used to:
Determine molecular mass of proteins or other biomolecules
switchSENSE-technology
switchSENSE-technology allows the qualitative and quantitative characterization of molecular interactions and binding affinities (as well as conformational changes of biomolecules). Using customizable DNA nanolevers on a chip surface the molecular interactions are monitored following changes in the fluorescent intensity of a dye attached to the nanolever (or biomolecule).
Typically used to:
- Characterize protein-protein/peptide/small molecule interactions
Characterization of Protein-RNA/DNA interactions
Surface Plasmon Resonance (SPR)/Biacore
SPR technology allows the qualitative and quantitative characterization of molecular interactions and binding affinities. It enables the kinetic, real-time and label-free analysis of biomolecule interactions (especially protein-protein interactions).
Typically used to:
- Characterize protein-protein interactions
Characterize the antigen binding properties of antibodies
SEC-MALS (combines multi-angle light scattering with size-exclusion chromatography)
SEC-MALS instruments allow to determine the weight-averaged molar mass of proteins and other macromolecules. The molecular mass is determined label free in solution by following the intensity of scattered light of the macromolecules separated on a SEC column.
Typically used to:
- Determine the molecular mass of proteins and protein complexes
Analytical separation and characterization of different protein complexes
Dynamic Light Scattering (DLS)
DLS measures the particle size of dispersed systems from sub-nanometer to several micrometers in diameter. This allows to analyze the particle distribution and composition of complex systems of macromolecules.
Typically used to:
- Determine the particle diameter and molecular mass (of proteins and other macromolecules)
- Determine particle distributions of a complex mixture (e.g. oligomer distributions of proteins)
Proteins in the Spotlight: Microscope Analysis, Life Cell Imaging, Confocal Scanning, High Resolution Imaging, Image Processing (AI) and High-Throughput Imaging
- Microscopy
- confocal microscopy
- spinning disc
- epifluorecent microscopy
- fluorescence-lifetime imaging microscopy (FLIM)
- total internal reflection fluorescence microscopy (TIRF)
- Fluorescence-activated cell sorting (FACS)
A deep dive into the proteome with greatest precision: Global Proteome Analysis, Whole Cell Proteomics, Protein Quantification, Protein Identification (Fingerprint, Full length, crosslinks), Protein Modifications, Protein dynamics, Small molecules, Protein-Protein Interactions, Target Proteins of Compounds, Binding Site Identification, Metabolomics
Trapped Ion Mobility Spectrometry Time of Flight Mass Spectrometry (TIMS TOF MS)
High end MS-setup used for proteome analysis. Especially suited to analyses highly complex samples and total cell extracts in comparative, qualitative and quantitative manner.
Typically used to:
- Compare the proteomes of an organism with and without drug/heat/xx treatment
- Identify and analyze interactions of proteins
- Identify PTMs
Quantify proteins in complex samples
Matrix-assisted Laser Desorption/Ionization Mass Spectrometry (MALDIMS)
With our MALDI setup we especially analyze the mass of Peptides, Proteins and other molecules in a mass range from ~500 Da to several thousand Da (in case up to 100 000 Da).
Typically used to:
- Determine the mass of peptides, proteins or other macromolecules (only in service mode)
- Identify proteins (MS fingerprint; only in service mode (here“PDF” for service))
Hydrogen Deuterium Exchange Mass Spectrometry (HDX-MS)
The automated hydrogen-deuterium-exchanges platform coupled with an automated high-resolution LC-ESI-TOF MS allows to kinetically analyse the dynamics of proteins as well as the monitoring of changes in the dynamics after binding of compounds or other proteins. Thereby changes in the uptake of deuterium (resulting in higher masses) are followed in the MS-system with peptide resolution.
Typically used to:
- Determine dynamic regions in proteins
- Comparing different mutants in terms of dynamics
- Identification of protein-protein binding sites
- Identify changes in dynamics upon ligand or partner binding
Liquid Chromatography / Mass Selective Detection Mass Spectrometry (LC/MSD, LC-MS)
Liquid Chromatography / Electrospray Ionisation (LC-ESI-MS)
Using exchangeable reversed phase LC setups (C18; C4) we apply our LC-ESI-MS systems to analyse the mass of small molecules (e.g. 100 – 2000 Da); Peptides (500 – 4000 Da) or proteins (5 – 100 kDa). MS-setups with different analysator techniques (TOF including ion mobility; Orbitrap-Tribrid; Orbitrag or classical ion traps) and distinguished setups allow also high resolution proteomics and metabolomics studies.
Typically used to:
- Determine the mass of (native, full-length) proteins, peptides, or other molecules
- Determine the high-resolution mass of (native, full-length) proteins (only in-service mode (here PDF for service))
- Determine the high-resolution mass of small molecules (only in-service mode (here PDF for service)
- Identification (quality control) of purified proteins and peptide mapping (only in-service mode (here PDF for service))
- Metabolomics
- Analysis of low complexity samples (e.g. identification of several proteins within one complex)
- High resolution analysis of highly complex samples; especially including low abundant proteins
- Single cell proteomics
- Crosslink analysis
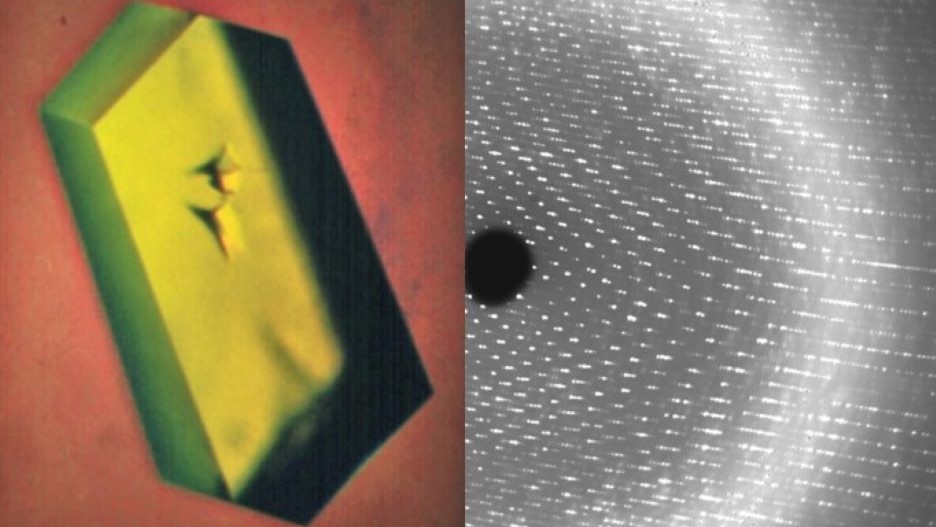
Structural characterization of biomolecules and their interactions at the atomic level using X-ray crystallography
• Robot systems Crystal Phoenix and Crystal Gryphon (Art Robbins Instruments) for high throughput screening of crystallization conditions
• Robot system Oryx4 (Douglas instruments) for microseeding
• Hamilton robot system for fine screening of crystallization conditions
• Glovebox with an OryxNano robot (Douglas instruments) for crystallization of oxygen sensitive proteins
For more details, please see https://www.bio.nat.tum.de/biochemie/infrastructure/
Access
Only registered members have access to the CPA Central Facilities. CPA members are automatically registered.
If you wish to register and submit for access, please send the filled template to CPA(at)tum.de
Terms of Use for CPA Users are available for registered users. CPA Central Booking System is available for registered users.